

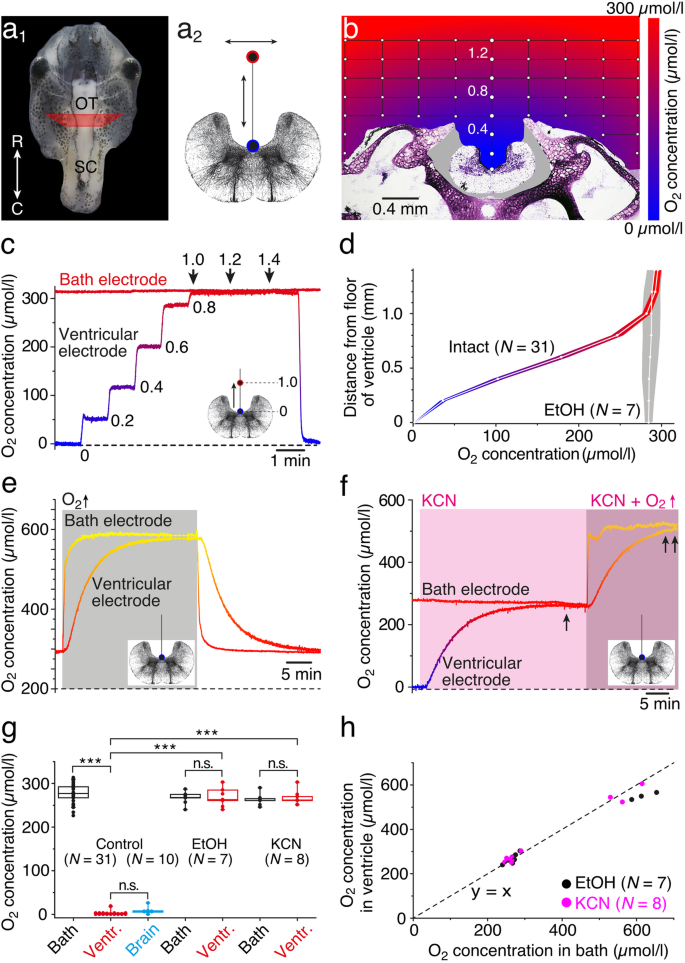
However, these efforts are still in the research stage, as their activity and stability are still lower than that of the Pt catalyst. Considerable research has been conducted to try to (1) reduce the costs of fuel cells, which is one of the stumbling blocks in fuel cell commercialisation using low-cost non-Pt catalysts such as supported platinum group metals Pd, Ir and Ru (2) improve the electrocatalytic activity of the cathode catalyst, which includes using bimetallic alloy catalysts, transition metal macrocyanides, transition metal chalcogenides and metal oxides in order to improve the ORR kinetics on the new catalyst and (3) fabricate Pt with novel nanostructures such as nanotubes, graphene and carbon nanofibres (CNFs), as it is known that supports may significantly affect the performance of the catalyst. These high potentials result in the formation of adsorbed species on a platinum surface that restrain the ORR and hence result in performance loss. It is also reported that Pt still shows over-potentials of over 400 mV from the equilibrium reversible potentials (1.19 V at 80☌). Pt is the electrocatalyst that is currently used for ORR reactions, as it is the only commercially available catalyst with sensible activity and stability for PEMFCs, although it offers limited commercialisation of fuel cells due to its limited availability and high cost. An electrocatalyst is used to induce a four-electron reduction of O 2 to water by utilising the protons that permeate from the anode. There are several issues that need to be addressed, including slow reaction kinetics at the cathode, which are due to highly irreversible ORR, and fuel crossover in the cathode, which causes a mixed potential, leading to potential loss and 25% reduction in efficiency, hence reducing the ORR performance. The kinetics of the ORR at the cathode are very important, as they are the factors for the performance of PEMFCs. The ORR is alkaline media versus reversible hydrogen electrode (RHE) at 25°, and its thermodynamic potentials at standard conditions are presented as follows :
In PEMFCs, a four-electron transfer is preferred. They suggest that ORR proceeds along two parallel reaction pathways with rates that are comparable.
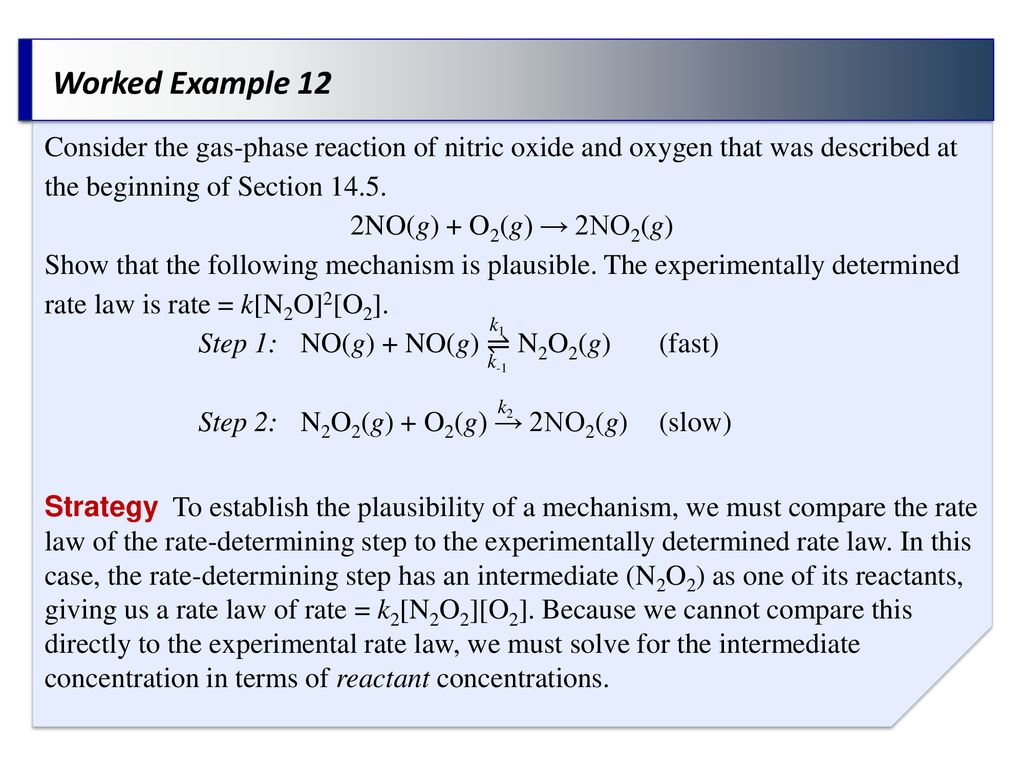

, making it easier to understand the complicated reaction pathway of oxygen on the metal surface. The most accepted mechanism of ORR was first proposed by Damjanovic et al. Oxygen reduction in aqueous solutions occurs mainly through two different pathways: either a four-electron reduction pathway from O 2 to H 2O or a two-electron pathway from O 2 to H 2O 2. This has led to more research being conducted in an effort to find alternate electrocatalysts that can be used. Among all catalysts evaluated, Pt is still the best catalyst for ORR. The major obstacle with Pt is that it belongs to the platinum group of metals, which are rare metals, hence too expensive for feasible commercialisation of fuel cells. ORR is the most important cathodic process in polymer electrolyte membrane fuel cells (PEMFCs). This is largely because ORR is of major importance to energy conversion, in particular in the field of fuel cells and metal-air batteries. Oxygen reduction reaction (ORR) has been the subject of extensive investigation over the last century.


 0 kommentar(er)
0 kommentar(er)
